Your One-stop Writing Workshop (at least for my classes)!
Constituents of Empirical Studies in More Detail

While the list above introduces the constituents contained within an empirical study, they all require more clarification.
Title Page First and foremost, make sure that any manuscript you turn in has a Title Page, that includes the title - which is a concise statement identifying the "variables or theoretical issues under investigation and the relationship between them," (APA, 2000, p. 23), your own name, and your institutional affiliation (see figure at right). For our class purposes, the author's note should include the names of your lab partners, and crediting me if you use my data in your report. Do note on the Title Page the words at top: "Running head: CALORIMETRY TO COMPARE THE ENERGY CONTENT OF FOODS." You will need to insert this on the Title page only, and on subsequent pages include only the title in caps, "CALORIMETRY TO COMPARE THE ENERGY CONTENT OF FOODS." Introduction The purpose of the introduction is to introduce the specific problem under study and describe the research strategy. You may want to consider these questions before writing, and answer in the body of your text:
Method This section describes in detail how your study was conducted. This should be so complete that anyone with your similar background could carry out the investigation. In this section you want to include all materials, equipment, chemicals and techniques used in the execution of your investigation. You may divide this into subsections, so that one section enumerates the materials you used, and another the protocol. If you performed two different types of experiments, these may similarly be divided into subsections. Do not include results in this section. As an example, do not say, "We measured the increase in temperature and found it to be 116 degrees Celsius." Instead say, "We noted the increase in temperature and recorded it in Table 1.3." 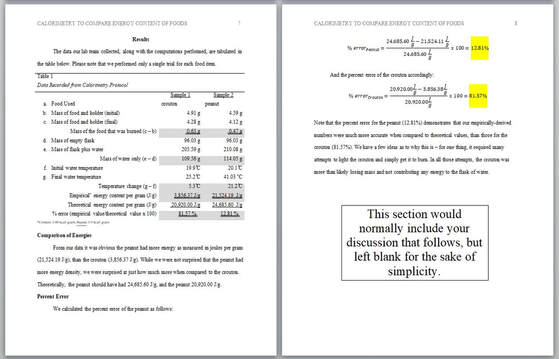
Results
Here you include measured values, calculated values, and statistically derived values. Feel free to include graphical representations of your data, such as data tables, pie charts, and graphs. You want to be sure to not merely present these visual elements, but describe them. As an example, in this calorimetry lab, you would not let your data table alone present your percent error for a peanut versus the crouton; make a statement: "Note that the percent error for the peanut (12.81%) demonstrates that our empirically-derived numbers were much more accurate when compared to theoretical values, than those for the crouton (81.57%)." Discussion After presenting your results, the discussion allows space to evaluate and interpret them. You might answer whether the results supported your original hypothesis? If not, why? What could you have done to improve on the experiment? What were the limitations of your study? Perhaps the study could have been improved by performing more than a single trial? You might state something such as, "Our calorimetric setup had many limitations, since much of the heat released by the burning food was not directed onto the flask, but lost to the surroundings." Simply put, the discussion section is where you simply discuss your results. References These acknowledge the work of scholars you cite, and provide a reliable means by which readers of your paper can locate their work. Start your reference list on a new page, and double-space all entries. You may want to include your textbook, it would appear as follows: References for course textbooks For General Chemistry labs: Wilbraham, A.C., Staley, D.D., Matta, M.S., & Waterman, E.L. (2002). Chemistry. Upper Saddle River, NJ: Prentice Hall, Inc. For Organic Chemistry labs: Karty, J. (2014). Organic Chemistry: Principles and Mechanisms. WW Norton & Company. For Advanced Physics labs: Wilson, J.D., Buffa, A.J., & Lou, B. (2007). College Physics, Sixth Edition. Upper Saddle River, NJ: Prentice Hall, Inc. Vinaja, S., Ridgely, J., Shoemaker, T., Smith, S., & McDivitt, B. (2017). Physics: The foundational science - laboratory manual. Pensacola, FL: A Beka Book. References for lab protocol handouts For General Chemistry Hydrometer/ Specific Gravity lab: Providence Extension Program/ General Chemistry course (2019). Using a Hydrometer to Determine Concentration of Sugar in a Commercial Soft Drink. Loveland, OH: Self-publish. For General Chemistry Calorimetry lab: Providence Extension Program/ General Chemistry course. (2018). Energy Content of Foods – Calorimetry. Loveland, OH: Self-publish. For Organic Chemistry Biodiesel lab: Loyola University of Chicago - Institute of Environmental Sustainability. (2017). Biodiesel Labs - Teacher Manual with Student Documents. Retrieved from: https://www.luc.edu/media/lucedu/sustainability-new/pdfs/biodiesel/Biodiesel%20Curricula%20-%20Version%205.0.pdf For Advanced Physics Hooke's Law lab: Providence Extension Program/ Advanced Physics course. (2018). Hooke's Law. Loveland, OH: Self-publish. Credits for this Webpage:
|
American Psychological Association. (2010). Publication Manual of the American Psychological Association, 6th Edition: American Psychological Association. American Psychological Association.


